A low-cost and novel nanomaterial to remove antibiotic: a solution for developing countries
Environmental pollution is one of the most pressing problems today, it threatens biodiversity, climate change and many other consequences that humans have to bear. Educating the young generation about environmental protection is a solution to protect the environment for the future. Environmental pollution is a problem not only in one region, but everywhere, in rural, urban, mountainous and coastal areas, especially water sources. The decontamination of antibiotics in soil and surface waters from natural environment is one of the emerging issues. Furthermore, an increase of animal protein in developing countries was demonstrated, led to the antibiotic resistance and antibiotic residues into food products. This work reported a facile investigation of the physicochemical characterizations of magnetic Fe/NH2/SiO2 (FNS) nanomaterial fabricated by the precipitation method and simple grafting with amino silica, and its application as removal of β-Lactam Ampicillin (AMP) in the solution. To observe the surface chemistry and physicochemical properties of FNS, the combination of various techniques, such as scanning electron microscopy techniques (SEM-EDX), TEM, XRD, and magnetic saturation (Ms) value was employed. The results obtained by UV-Vis absorption method, zeta potential calculation indicated that the AMP adsorption efficiencies by FNS were stable and desired at acidic pH solutions, and optimum temperature of 30 °C.
This work was conducted and examined at VNU University of Education (UEd), Hanoi, Vietnam, in the research group of corresponding authors Professor Eldon R. Rene4,*, and Dr. Hoang Thu Ha1,*, Tran Dinh Minh1,*, with other co-authors such as: Ta Minh Trang2, Vu Dang Hoang Hiep2, Tran Long Vu2, Le Anh Duc3, Kim Thang Long5. 1,*VNU University of Education (UEd), Hanoi, Vietnam; 2High School for Gifted Students (HSGS), VNU University of Science; 3Cau Giay High School, Hanoi, Vietnam; 4,*UNESCO-IHE, Institute for Water Education, Delft, The Netherlands; 5Hanoi-Amsterdam High School for the Gifted, Hanoi, Vietnam. The authors synthesized the FNS according to both iron salts such as Fe3+ and Fe2+. After that aqua amonia was added in the system, and increasing the pH value up to 11. Then, the polymer APTMS was linked with iron oxide to form Fe/NH2 with the help of sonicator. Furthemore, the iron oxide amine was connected with Si2O to form the final product: FNS. AMP is a kind of beta – lactam, penicillin A group. On its structure had several Nitro-atoms in the amine groups such as NH2.
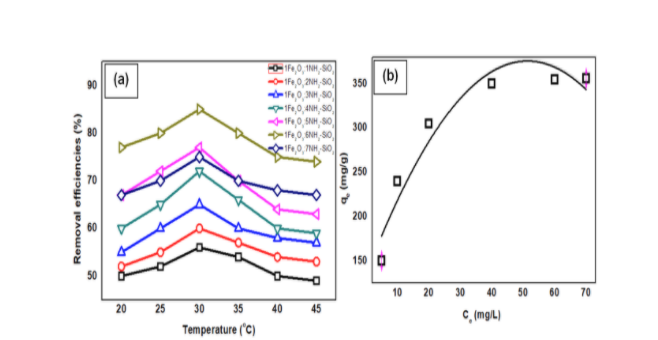
As can be seen form the figure of (a) Removal percentages of AMP on various ratio (w/w %) of synthesized FNS (conditions: C0: 20 ppm, m FNS: 20 mg, time: 20-45 °C, pH = 4, and V: 20 mL), and (b) Langmuir model curve of AMP adsorption isotherm (concentration ranged of 20-150 mg/L, time = 140 min at RT). Optimum temperature was to be found at 30 oC, and the adsorption was well-followed by Langmuir equation. In this work the AMP adsorption on FNS from aqua environment was examined with different ratio of iron oxide and amine modified-SiO2. FNS could be suggested as an effective material for antibiotic removal from solution, regarding on the maximum adsorption efficiency, and facile fabrication.
Author: Tran Dinh Minh, Ph.D.
Deputy Head of Political-Students Affairs Department;
Department of Educational Technology;
Secretary of Ho Chi Minh Communist Youth Union; High School of Education Sciences (HES);