Arsenic (III) contamination for the nearby ecosystem, caused toxic effects on the nervous, digestive and immune systems, and on lungs, kidneys, skin and eyes. With all the factors, we have mentioned above, are the reasons and motivations for our research in this topic, to solve the problems that arsenic has caused.
Arsenic contamination has made the ecosystem toxicity and human health, As(III) also destroyed the DNA and had the cancer potentials. Among the water purification techniques for removing a wide range of compounds from industrial wastewater, we have chosen the adsorption method because it is affordable, fast, simple to design, efficient and meets the great attention from the researchers. A pilot system was conducted in the University of Education (UEd) laboratory, Vietnam National University, accordingly: Research group: Truong Ngoc Kiem; Hoang Thu Ha; Vu Minh Anh; Nguyen Thi Hoa; Nguyen Thi Khanh Ly; Vu Thao Van; Nguyen Dang Quang; Hoang Huy Khanh; Le Huy, Nguyen Bao Huy; Manh Hoang Anh; Tran Chau Anh; Tran Hai Anh; Tran Dinh Minh*.
Affiliation: Vietnam National University, University of Education, Hoang Liet School, Hanoi-Amsterdam High School for the Gifted, Chu Van An High School, Marie Curie Hanoi School, and High School of Education Sciences.
In this study we investigated a novel and low-cost alkaline and acidic functional groups modified bamboo charcoal by using NH4OH and HCl solution (BC-N and BC-H) in nitrogen atmosphere heat treatment, for the arsenic adsorption from wastewaters.
To determine the adsorbents characterizations, SEM, TEM, FTIR and
XRD tools were evaluated.
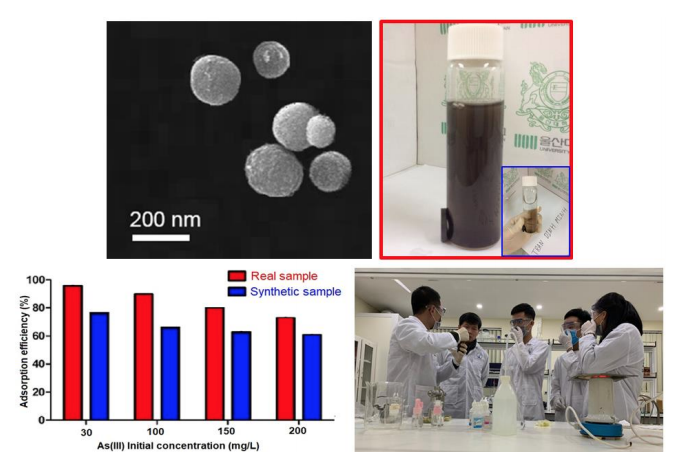
In the TEM analysis, it was indicated that BC-N had a nano scale structure, and BC-N had a spherical shape and homogeneous distribution.
The modified BC was synthesized by soaking BC into the aqua NH4OH and HCl solution solutions, then they were heated in the N2-atmosphere at different temperatures. The BC-N and BC-H indicated a higher As removal efficiency, as compared with the original BC. The optimal pH for As(III) adsorption was determined at 4. The As removal was mainly dependent on the initial concentration and adsorbent dosage. The As adsorption was well followed by the pseudo-second-order kinetic model and Freundlich isotherm.
Furthermore, As(III) adsorption efficiencies were decreased in real wastewater samples, as compared with the laboratory synthetic samples, in all samples of As(III)b initial concentrations. BC-N should be regarded as a promising adsorbent to remove As(III) in waters.
The modified BC-N and BC-H is a promising adsorbent for the treatment of arsenic- contaminated wastewaters. This project had demonstrated the research on the modified bamboo charcoal for fast adsorption to remove As(III) from waters. NaOH solution was selected for the suitable solvent to regenerate the reused BC-N.
The optimum conditions for the As(III) adsorption process were calculated: pH = 4, adsorbent dosage = 2.5 g/L.
This research was granted and funded by Vietnam National University, Hanoi, Vietnam.
Author: Tran Dinh Minh