Leishmaniases are a group of infectious and non-contagious severe parasitic diseases, caused by protozoans of the Leishmania genus. The transmission occurs through blood meal of infected female sand flies of the genera Phlebotomus, that are considered as intermediate hosts of these parasites. The flagellate promastigote form of the parasite is present in the sand fly, and once engulfed by host macrophages, it
converts into the aflagellate amastigote form (1,2).
Biological methods of nanoparticles (NPs) synthesis using microorganisms (3,4), enzymes (5), fungus (6), and plants or plant extracts (7,8) have been suggested as possible eco-friendly alternatives to chemical and physical methods. The development of green processes for the synthesis of nanoparticles is evolving into an important branch of nanotechnology especially silver nanoparticles, which have many applications (9-11). Chemical synthesis methods lead to presence of some toxic
chemical absorbed on the surface that may have adverse effect in the medical applications. Green synthesis provides advancement over chemical and physical method as it is cost effective, environment friendly, easily scaled up for large scale synthesis and in this method there is no need to use high pressure, energy, temperature and toxic chemicals (12,13).
Silver nanoparticles play a profound role in the field of biology and medicine due to their attractive physiochemical properties. Silver products have long been known to have strong inhibitory and bactericidal effects, as well as a broad spectrum of antimicrobial activities, which has been used for centuries to prevent and treat various diseases, most notably infections (14).
The particle size of NPs is play an important role in antimicrobial efficiency (15), so the strongest effect of the smallest sized of NPs showed more than the largest sized (16). The growth of some parasites such as Giardia, Leishmania, Plasmodium, Toxoplasma and insect larva is inhibited with the presence of silver nanoparticles, gold, chitosan, and oxidized metals (17).
Materials and Methods:
Preparation of AgNPs-TA
A 5 mL of Tannic Acid (TA) were added on 5 mL of AgNO 3 at 25 °C in conical flask.
The mixture was kept under strong magnetic stirring for 5 hours, until a brown colloidal dispersion of AgNPs-TA was seen and kept from light until use.
Characterization of nanoparticles
UV-Vis spectra were managed in absorbance mode (range 200-900 nm) with a double
beam to check the formation of nanoparticles. The particle size, shape and
morphology were evaluated by scanning electron microscopy (SEM) images.
Promastigotes preparation
Amastigotes of L. major which isolated from human skin lesions were cultured on Novy‐MacNeal‐Nicolle (NNN) medium containing agar (4 mg/100 ml) and defibrinated rabbit blood (10%). After transformation of amastigotes to promastigote‐like forms, the growth of promastigotes was continued in RPMI 1640 culture medium containing 100 μg/ml penicillin,100 μg/ml streptomycin supplemented with 10% fetal calf serum (FCS) (Gibco Co.) in a 28 °C incubator for two weeks. During several
passages the parasite suspension was precipitated in a refrigerated centrifuge for 10 minutes at 4500 rpm, and the supernatant was removed. Thus, the parasites achieved a fixed growth phase (stationary‐phase) after 12 days, and were ready for CL injection and induction (18, 19).
Experimental groups
The mice infected with L. major were randomly divided into three groups, each consisting of 10 mice as follows:
Group 1 with no treatment, was the control group.
Group 2, received AgNPs at a dose of 2 mg/kg under anesthesia (20).
Group 3, received AgNPs-TA at a dose of 2 mg/kg under anesthesia.
All above treatment were given to the lesions on days zero, 4, 8, and 12.
The parameters for evaluating treatment efficacy
The treatments efficacy were recorded according to the changes in the lesion and spleen parasite burden. The sizes of lesions were measured twice a week. The mice
were sacrificed after the first treatment and taken their spleen to determine the number of viable parasites (21). The spleens of the animals were placed into 6‐well plates containing complete RPMI‐1640 medium. After homogenization of the spleen tissue, the content of all wells was completed to 200 microliters with complete RPMI‐1640
medium. All samples were divided in three replicates. The prepared plates were put in incubator at 28 °C for 10 days. At last, the positive and negative results were reported in serial dilutions on the basis of the microscopic observations.
Results and Discussion:
The development of reliable and eco-friendly process for the synthesis of metallic nanoparticles is an important step in the field of application of nanotechnology. Some toxic chemical materials absorbed on the surface that may have side effect in the medical applications when used the chemical synthesis methods. Green synthesis prefer more than the chemical and the physical method as it is cost effective, environment friendly, easily scaled up for large scale synthesis and in this method
there is no need to use high pressure, energy, temperature and toxic chemicals.
Therefore, the production silver nanoparticles by biological methods of plant extract instead of other toxic methods was the best and most effective (22,23).
The absorption bands of some metallic nanoparticles in the visible region were shown due to collective oscillation of electrons in resonance with incident electromagnetic radiation, termed surface plasmon resonance (SPR) band (24).
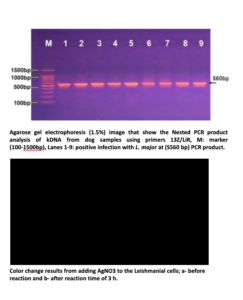
Sixty skin samples of suspected cutaneous leishmaniasis patients were detected for Leishmania amastigotes by microscopic observation out of which, 45 (75%) were positive; however, the NNN culture led to the growth of promastigotes in 42 samples (70%). Also, the results showed that 54 (90%) of samples were positive by PCR method ; 60% L.major and 30% L.tropica.
Leishmania major cells when exposed to silver ions showed a distinct and fairly broad UV–Vis absorption band centered at 425 nm because this nanoparticle was well dispersed without aggregation. The appearance of this band, which was assigned to a surface plasmon, is well documented for various metal nanoparticles with sizes ranging from 2 to 100 nm (25). SEM showed the formation of silver nanoparticles with an average size of 35 to 40 nm with inter-particle distance. UV-Vis absorption spectra have been proved to be quite sensitive to the formation of silver colloids
because silver nanoparticles exhibit an intense absorption peak due to the surface plasmon excitation (26,27).
The appearance of parasites in the presence of AgNPs-TA suggests some possible mechanisms of action. Previously literature, report that silver attacks bacteria’s cell membrane surface, altering permeability, osmotic equilibrium and thus, metabolic pathways of the cell. Silver nanoparticles may also bind to the DNA, preventing replication (28-32).
TA-coated gold and silver nanoparticles SPR bands were red-shifted
compared to values reported in literature, 520 and 380-450 nm, respectively
(33,34). This bathochromic effect is likely caused by the supramolecular
interaction between the nanoparticles and the surface-protecting TA
polyphenol, altering the electron transfer energy. Besides shape, size and
type of the material, the position of the SPR band is highly dependent on the
dielectric constant of the surrounding medium, once it changes the resonance frequency of the electrons onto NMNPs’ surface (35-37).
Conclusions:
Silver nanoparticles synthesized using tannic acid have potential application as an antileishmanial treatment. Furthermore, the use of natural compounds involves to the development of clean, nontoxic, biocompatible and environmentally benign methods to synthesize noble metal nanoparticles.
References :
1. Carneiro SMP, Carvalho FAA, Santana LCLR, Sousa APL, Moita-Neto JM
and Chaves MH. The cytotoxic and antileishmanial activity of extracts and
fractions of leaves and fruits of Azadirachta indica. Biol Res., 2012; 45: 111-
116.
2. Kevric I, Cappel MA and Keeling JH. New World and Old World Leishmania
Infections: A Practical Review. Dermatol Clin., 2015; 33: 579-593.
3. Klaus T, Joerger R, Olsson E, Granqvist CG. Silver-based crystalline
nanoparticles, microbially fabricated. J. Proc. Natl. Acad. Sci.USA., 1999; 96 :
13611-13614.
4. Konishi Y, Uruga T . Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J Biotechnol ., 2007; 128:648-653.
5. Willner I, Baron R, Willner B. Growing metal nanoparticles by enzymes. J.
Adv. Mater., 2006; 18:1109-1120.
6. Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paraliker
KM, Balasubramanya RH. Biological synthesis of silver nanoparticles using
the fungus Aspergillus flavus. Mater Lett., 2007; 61:1413-1418.
7. Shankar SS, Ahmed A, Akkamwar B, Sastry M, Rai A, Singh A . Biological
synthesis of triangular gold nanoprism. Nature. 2004; 3:482.
8. Ahmad N, Sharma S, Singh VN, Shamsi SF, Fatma A, Mehta BR.
Biosynthesis of silver nanoparticles from Desmodium triflorum: a novel
approach towards weed utilization. Biotechnol. Res. Int., 2011; 454090 (1-8).
9. Armendariz V, Gardea-Torresdey JL, Jose Yacaman M, Gonzalez J, Herrera I,Parsons JG . Proceedings of Conference on Application of Waste Remediation Technologies to Agricultural Contamination of Water Resources. Kansas City Mo, USA. 2002.
10. Kim BY, Rutka JT, Chan WC. Nanomedicine. N. Engl. J. Med., 2010; 363
(25): 2434-2443.
11. Kyriacou SV, Brownlow WJ, Xu XN . Using nanoparticle opti chitosan assay for direct observation of the function of antimicrobial agents in single live bacterial cells. Biochemistry. 2004; 43:140-147.
12. Singh A, Jain D, Upadhyay MK, Khandelwal N, Verma HN . Green synthesis of silver nanoparticles using Argimone mexicana leaf extract and evaluation of their antimicrobial activities. Digest J. Nanometer. Biostruct., 2010; 5 (2):
483-489.
13. Jain D, Kumar Daima H, Kachhwaha S, Kothari SL. Synthesis of plant-
mediated silver nanoparticles using Papaya fruit extract and evaluation of their antimicrobial activities. Digest J. Nanomater. Biostruct., 2009; 4(3):557-563 .
14. Shankar, S.S., Rai, A., Ankamwar, B., Singh, A., Ahmad, A., Sastry, M.. Nat.
Mater., 2004; 3, 482–488.
15. Adams, LK, Lyon, DY, Alvarez, PJ. Comparative eco-toxicity of nanoscale
TiO2, SiO2, and ZnO water suspensions. Water Res., 2006; 40: 3527-32.
16. Lu, Z, Rong, K, Li, J, Yang, H, Chen, R. Size-dependent antibacterial
activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J.
Mater. Sci. Mater. Med., 2013; 24:1465-71.
17. Elmi, T, Gholami, S, Fakhar, M, Azizi, F. A Review on the most of
nanoparticles in the treatment of parasitic infections. J. Mazand. Univ. Med.
Sci. 2013; 23, 102:126-33.
18. Sazgarnia A, Zabolinejad N, Layegh P, Rajabi O, Berenji F, Javidi Z, et al.
Antileishmanial activity of liposomal clarithromycin against Leishmania
major Promastigotes. Iran J Basic Med Sci 2012; 15:1210‐1214.
19. Mohebali M, Rezayat MM, Gilani K, Sarkar S, Akhoundi B, Esmaeili J, et al. Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study. DARU
2009;4:285‐289.
20. Xue Y, Zhang S, Huang Y, Zhang T, Liu X, Hu Y, et al. Acute toxic effects
and gender‐related biokinetics of silver nanoparticles following an intravenous injection in mice. J Appl Toxicol 2012; 32:890‐899.
21. Buffet P, Sulahian A, Garin Y, Nassar N, Derouin F. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected
mice. Antimicrob Agents Chemother 1995; 39:2167‐2168.
22. Jain D, Kumar Daima H, Kachhwaha S, Kothari SL . Synthesis of plant-
mediated silver nanoparticles using Papaya fruit extract and evaluation of their antimicrobial activities. Digest J. Nanomater. Biostruct., 2009; 4(3):557-563 .
23. Vyom P, Rashmi P, Bechan S, Avinash CP . Parthenium leaf extract mediated synthesis of silver nanoparticles: a novel approach towards weed utilization. Digest J. nanometer. Biostructures., 2009; 4(1):45-50.
24. Melo JR MA, Santos LSS, Goncalves MC and Nogueira AF. Preparação de
nanopartículas de prata e ouro: um método simples para a introdução da
nanociência em laboratório de ensino. Quim Nova., 2012; 35: 1872-1878.
25. El-Raheem A, El-Shanshoury AR, ElSilk SE, Ebeid ME. Extracellular
biosynthesis of silver nanoparticles using Escherichia coli ATCC 8739,
Bacillus subtilis ATCC 6633, and Streptococcus thermophilus ESh1 and their
antimicrobial activities. ISRN Nanotechnol.,12011; -7.
26. Gao, J., K. Powers, et al. "Influence of Suwannee River humic acid on particle properties and toxicity of silver nanoparticles." Chemosphere., 2012; 89 (1):
96-101.
27. Abdulsadah A. Rahi1, Magda A. Ali and Alaa H. Al-Charrakh. Biosynthesis of silver nanoparticles by Leishmania tropica. Afr. J. Biotechnol., 2013; 12
(48): 6718-6722.
28. Jebali A and Kazemi B. Nano-based antileishmanial agents: A toxicological study on nanoparticles for future treatment of cutaneous leishmaniasis. Toxicol In Vitro. 2013; 27: 1896-1904.
29. Chen M, Yang Z, Wu H, Pan X, Xie X and Wu C. 2011. Antimicrobial
activity and the mechanism of silver nanoparticle thermosensitive gel. Int J
Nanomed 6: 2873-2877.
30. Perni S, Piccirillo C, Pratten J, Prokopovich P, Chrzanowski W, Parkin IP and Wilson M. The antimicrobial properties of light activated polymers containing methylene blue and gold nanoparticles. Biomaterials. 2009; 30: 89-93.
31. Allahverdiyev AM, Abamores, Bagirova M, Ustundag CB, Kaya C Kaya F
and Rafailovich M. Antileishmanial effect of silver nanoparticles and their
enhanced antiparasitic activity under ultraviolet light. Int J Nanomed., 2011; 6: 2705-2714.
32. Duran N, Marcato PD, Contird, Alves OL,Costa FTM and Brocchi M.
Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J Braz Chem Soc., 2010; 21: 949-959.
33. Sharifi N, Dabirian A, Danaei D and Taghavinia N. Aggregates of
plasmonic nanoparticles for broadband light trapping in dye-sensitized
solar cells. J Opt-UK., 2015;18: 1-7.
34. Silva ATB, Coelho AG, Lopes LCS, Martins MVA, Crespilho FN,
Merkoci A and Silva WC. Nano-assembled supramolecular films from
chitosan-stabilized gold nanoparticles and Cobalt (II) phthalocyanine. J
Braz Chem Soc., 2013; 24: 1237-1245.
35. Song and Jing. Insight into the localized surface plasmon resonance
property of core-satellite nanostructures: Theoretical prediction and
experimental validation. J Colloid Interf Sci., 2017; 505: 373-382.
36. Martinsson, Otte MA, Shahjamali MM,Sepulveda B and Aili D.
Substract effect on the Reffractive Index sensity of silver nanoparticles.
J Phys Chem C., 2014; 118: 24680-24687.
37. Mahmoud MA, Chamanzar M, Adibi A and Elseyed MA. Effect of the
dielectric constant of the surrounding medium and the substrate on the
surface plasmon resonance spectrum and sensitivity factors of highly
symmetric systems:silver nanocubes. J Am Chem Soc., 2012; 134:
6434-6442.
Abdulsada A. Rahi 1* , Magda A. Ali 2 and Zaid A. Abdulabbas ( Department of Biology, College of Science, Wasit University, Kut, Iraq.)
(College of Medicine, Wasit University, Kut, Iraq)