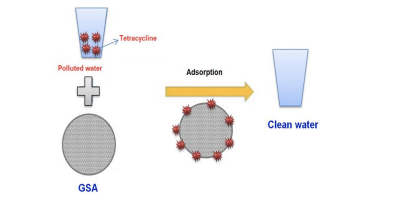
Tetracycline removal from the synthetic wastewater samples in VNU-University of Education, Hanoi, Vietnam.
In this research, we offered three main ideas, firstly, GSA was fabricated by graphene oxide (GO) grafted with Tin(II) oxide (SnO) wet prepared in the framework of activation procedure. Secondly, the TC adsorption ability was calculated by several methods in the views of pH, initial concentration, TEM, porous textural and temperature. Finally, the optimum conditions for tetracycline removal was observed: pH = 4, To=30 oC and Qmax=1201mg/g. Keywords: Tetracycline removal; Nanocomposite; Antibiotic; Graphene oxide. Tetracycline antibiotics was proven by many studies to have dangerous effect on human body such as fatty liver, acute pancreatitis, severe hepato-nephrotoxicity, kidney damage and insipidus diabetes. Therefore, this compound toxicity had become the reason and motivation for our group to research in novel fabrication of advanced nanocomposite to remove 2 tetracycline antibiotic from natural environment. Based on the experiments, our group has found the solution in which to remove the TC out of the polluted water from all sources using a compound named GSA. After researching for a long time we have found out the solution in which to remove the TC out of the polluted water from all sources. By seeing from the illustrative image that we have shown above in the poster, the adsorption method of the TC was initiated. We use GSA in which is the GO/Sno/AP nanocomposite. After conducting, TC molecules were attached on the GSA surface. In the end, the unpolluted water which contained TC were disinfected.
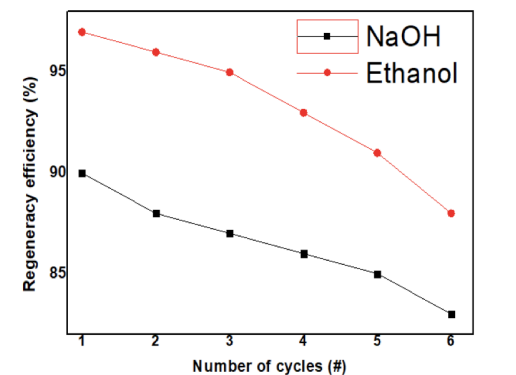
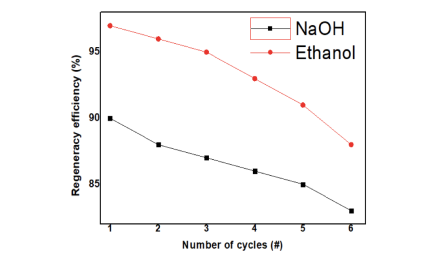
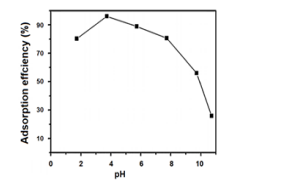
GSA which contained graphene oxide (GO),tin(II) oxide (SnO), and AP (amino polymer) was put into preparation. Through a few of the chemical modifications, Sno didn’t change rapidly, and the colour was still black in order to adsorp in the best way. In the following step we will introduce furthermore about the method that we used to treated the polluted water. The Sno nanocomposites were synthesized using semisolvothermal reaction technique. These nanocomposites were arranged using different combinations of solvents such as ethanol, water, and ethylene glycol at 180 °C for 24 h. Through few of the chemical modification, the SnO didn’t change rapidly. 3 Due to the adsortivity of the GSA surface, the material was able to be regenerated through time, which would conserve the resources and protect the environment. By using the ethanol treatment as shown in the illustrative image, the ethanol above 90% can be used to regenerate the GSA upon the cycles. Through each cycle, the efficiency will drop down frequently. In the 1st cycle, the frequency was very high and reach to above 97% efficiency After that, the efficiency drop down rapidly and to the 5th cycle the GSA couldn’t be treated again. The optimal pH level for the adsorption efficiency of GSA to reach maximum, specifically 94%, was at 4. After pH 4, the efficiency decreased progressively: pH 6 (88%), pH 8 (81%), pH 10 (57%).
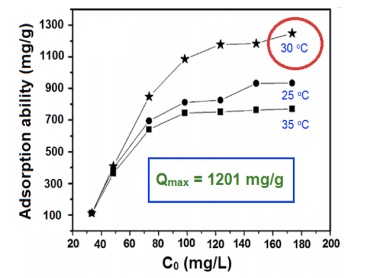
The results of the experiment depended on the temperature and the concentration of the adsorbate Tetracycline. According to the study, the intial concentration ( C0 ) is directly proportional to the adsorption ability of GSA: when C0 increased from 40 to 80 mg/L, the adsorption efficiency rose upwardly; then the increase in adsorption became less as the C0 rose from 80 to 130 mg/L, suggesting that there is a limit of adsorption that GSA couldnt reach over despite how large the concentration of Tetracycline was. Furthermore, as the temperature was looked into, the diagram showed that among 25°C, 30°C, and 35°C, the optimal temperature for the adsorption is 30°C (when the adsorption ability reach the highest with Qmax =1201 mg/g). This concluded that the temperature and initial concentration do 4 affect the Tetracycline adsorption efficiency of GSA. This work was conducted and examined at VNU University of Education (UEd), Hanoi, Vietnam, in the research group of corresponding authors Professor Eldon R. Rene6,*, and Tran Dinh Minh1,*, with other coauthors such as: Hoang Thu Ha1 ; Hoang Ly Bao Long2 ; Hoang Ly Tuan Long3 ; Le Huy4 ; Dao Quang Minh5 , Le Ha Khoa5 , Eldon R. Rene6 . 1VNU University of Education (UEd), Hanoi, Vietnam; 2High School for Gifted Students (HSGS); 3The Olympia Schools; 4HanoiAmsterdam High School for the Gifted; 5High School of Education Sciences (HES-UEdVNU); 6UNESCO-IHE, Institute for Water Education, Delft, The Netherlands.
In 2013 and 2015, there had been research on solutions to remove tetracycline (Fe-AC with Qmax= 769 mg/g and Petrolium Coke-AC with Qmax = 1121 mg/g), yet, GSA had performed the best, displaying the largest value for TC removal with Qmax= 1201 mg/g. This concluded that GSA is the most promising adsorbent for tetracycline, up to date. The novel fabrication of advanced nanocomposite to remove tetracycline antibiotic from the natural environment afforded the following conclusion: 5 + With the benefits of high BET surface area, and well developed porous texture, the maximum absorption magnitude of TC on GSA reached to 1201 mg/g. + The GSA after TC absorption has the option to reuse by treating with C2H5OH and through this project, we have demonstrated the possibility of advanced nanocomposite to eliminate tetracycline antibiotics from the natural environment. In conclusion, with the benefits of high BET surface area, and well developed porous texture, the maximum absorption magnitude of TC on GSA reached 1201 mg/g, it is likewise discovered that the GSA after TC absorption has the option to reuse by treating with C2H5OH. Last but not least, through this project, we have demonstrated the possibility of advanced nanocomposite to eliminate tetracycline antibiotics from the natural environment, we have gathered some various information as shown in the preferences.